Water Treatment
Water Hardness and Calcium Removal Methods


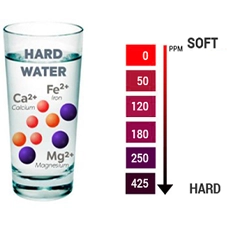
Water hardness refers to the amount of dissolved minerals, primarily calcium and magnesium, present in your water supply. While generally not a health hazard, hard water can cause some inconveniences and potential issues.
Here's a breakdown of water hardness and its effects:
Minerals and Hardness
Calcium and magnesium are naturally occurring minerals found in rocks and soil. When water percolates through these layers, it picks up these minerals, leading to hard water.
Impact on Daily Life
Hard water can make it difficult to lather soap and shampoo, leaving a filmy residue. It can also lead to scale buildup in pipes, appliances like kettles and coffee makers, reducing efficiency and shortening their lifespan. Here's a clearer picture of concerns
Skin and Hair
Hard water can be drying to the skin and scalp, potentially exacerbating dryness or eczema. It can also make hair feel dull and difficult to manage.
Appliance Issues
Scale buildup from hard water can clog pipes, reduce water flow, and shorten the lifespan of appliances that use water heating elements.
Soap Scum
Hard water interferes with the ability of soap and detergents to lather effectively, leading to higher usage and potential soap scum buildup.
Potential Concerns
There's some debate about the health effects of hard water. While some studies suggest a link between hard water and certain skin conditions like eczema, the World Health Organization (WHO) states there's no definitive evidence of health risks associated with drinking hard water.
Decalcification, also referred to as water softening, is the process of removing hardness-causing minerals, primarily calcium and magnesium, from water. Hard water can lead to scale buildup in pipes and appliances, reduced efficiency, and soap scum. Here are some common solutions for decalcification. Choosing the right method depends on the level of water hardness, consumption needs, budget, and available technology.
- Ion Exchange
- Distillation
- Reverse Osmosis
- Chemical treatment
- Emerging technology
Ion Exchange
This method utilizes resin beads that attract and trap calcium and magnesium ions as water passes through. The captured ions are replaced with sodium ions, resulting in softened water. Ion exchange systems are popular for whole-house water softening and require regeneration with salt solution periodically

- Resin Beads: The core of an ion exchange system is a tank filled with tiny resin beads. These beads have a permanent electrical charge and attached charged groups (functional groups) that attract ions of the opposite charge. For water softening, the resin beads typically have negatively charged functional groups.
- Brine Tank (in some models): This tank holds a salt solution (brine) used to regenerate the resin. During regeneration, the brine solution flushes out the calcium and magnesium ions trapped by the resin, restoring its capacity for softening.
- Attraction and Replacement: When hard water flows through the tank, calcium (Ca²+) and magnesium (Mg²+) ions, with their positive charges, are attracted to the negatively charged functional groups on the resin beads. These ions exchange places with harmless sodium (Na+) ions already attached to the resin. The softened water exiting the tank now contains sodium ions instead of calcium and magnesium ions.
- Regeneration: Over time, the resin beads become saturated with calcium and magnesium ions and need to be regenerated. This process involves flushing the tank with a concentrated salt solution (typically brine). The high concentration of sodium ions displaces the calcium and magnesium ions from the resin beads, restoring the resin's capacity for softening.
- Control Valve: This valve regulates the flow of water through the system. It directs the hard water to be softened through the resin tank, then diverts the softened water out to the house plumbing, and also initiates the regeneration cycle when necessary.
- Distributor: This component ensures even water distribution throughout the resin bed in the tank to optimize softening performance.
- Mechanism: Uses ion exchange resins to replace calcium and magnesium ions with sodium ions.
- Advantages:
- Efficient and cost-effective
- Low maintenance
- Does not change the taste or smell of water
- Disadvantages:
- Requires salt for regeneration
- May increase sodium levels in water
- Not suitable for industrial applications
2. Distillation:
Distillation is a versatile method for purifying water, making it useful not just for decalcification but also for removing other contaminants like bacteria, viruses, and minerals. The boiling process vaporizes water, leaving behind dissolved minerals. Distilled water is the softest form of water achievable, but it also lacks beneficial minerals and can taste bland. Distillation is suitable for small-scale applications like steam irons.

- Boiling and Evaporation: In a distillation unit, water is heated to its boiling point. As the water boils, it transforms into vapor, leaving behind dissolved minerals and impurities in the heating chamber.
- Condensation: The water vapor then travels to a separate chamber where it's cooled. This cooling process causes the vapor to condense back into liquid form as distilled water. Since the minerals have a higher boiling point than water, they remain in the heating chamber.
- Collection: The condensed distilled water is collected in a separate container. This water is now free from most impurities and minerals, including hardness-causing calcium and magnesium.
- Pre-treatment System: This section filters out impurities like chlorine, turbidity, and organic matter that can damage the desalination membranes or hinder the desalination process.
- Membrane Assembly: This is the core of the desalination system, where the separation of salt from water occurs. Depending on the desalination method, different types of membranes are used, such as reverse osmosis membranes in RO desalination.
- High-Pressure Pumps: These pumps pressurize the seawater or brackish water feed stream before it enters the membrane assembly. This pressure is necessary to overcome the natural osmotic pressure that pushes water towards the saltier side of the membrane.
- Post-treatment System: In some desalination processes, the desalinated water may require further treatment to adjust its mineral content or pH level to make it suitable for drinking or irrigation.
- Mechanism: Boils water and converts the resulting steam into purified water.
- Advantages:
- Removes almost all impurities including calcium
- Suitable for industrial and laboratory applications
- Disadvantages:
- Expensive and energy-intensive
- Changes the taste of water
- Leaves mineral deposits in the tank
3. Reverse Osmosis
Reverse osmosis is a water purification process that uses semi-permeable membranes to separate impurities, including calcium, from water. In this process, pressurized water passes through a membrane that allows water molecules to pass through while rejecting calcium ions and other impurities. The membrane selectively allows smaller water molecules to pass through its pores, while larger calcium ions and other contaminants are blocked due to their size. This process effectively removes calcium from water by up to 99%. However, reverse osmosis membranes require periodic maintenance and replacement.
- Residential Reverse Osmosis Systems:These systems are designed to be installed under the kitchen sink and provide purified water for drinking, cooking, and other household uses.
- Industrial Reverse Osmosis Systems:These systems are used to treat large volumes of water for industrial and commercial applications such as food and beverage production, pharmaceutical manufacturing, and laboratory testing.
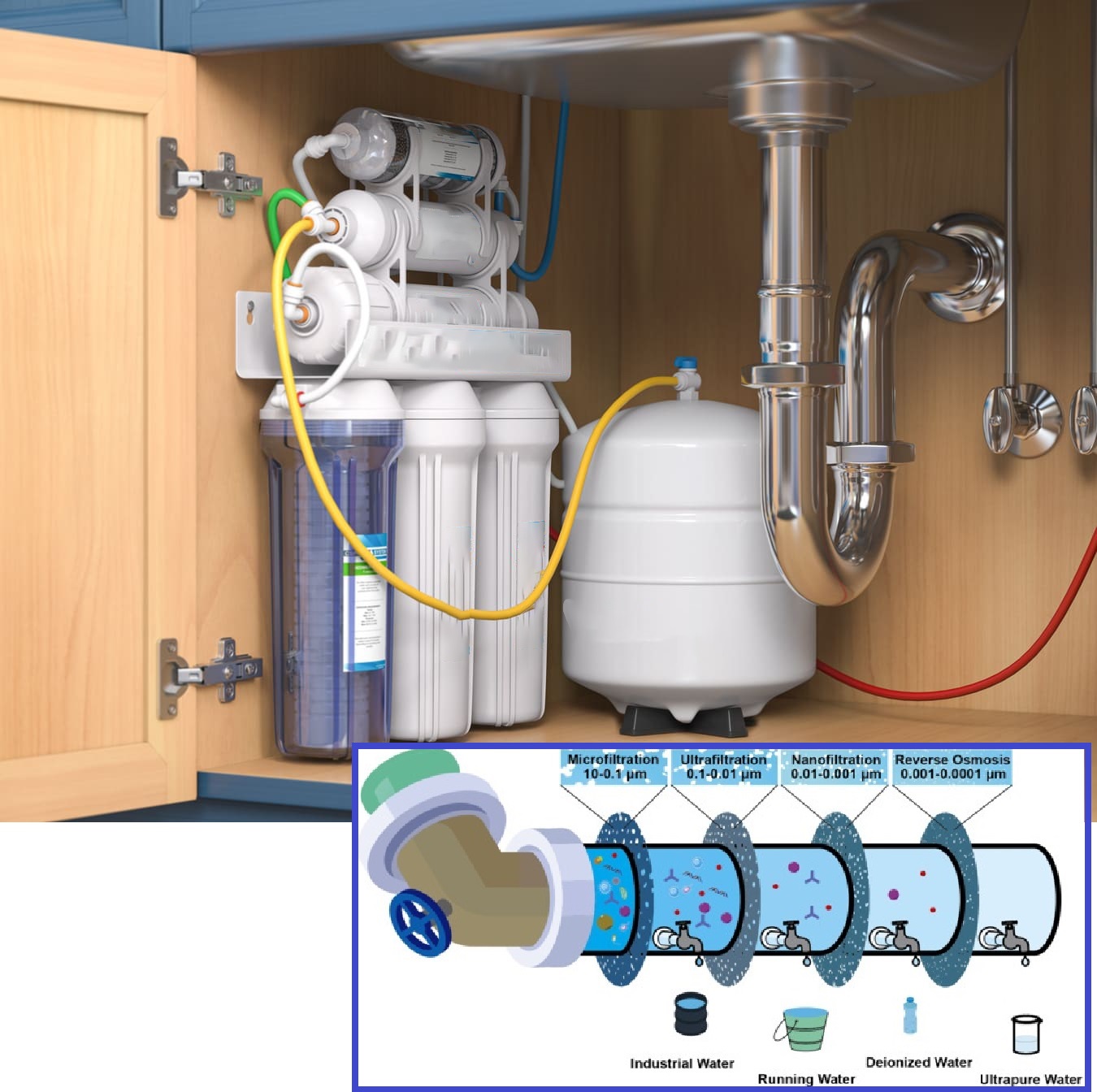
1.Pre-filters:
- These are typically sediment and carbon filters.
- The sediment filter removes physical impurities like dirt, sand, and rust particles that could damage the RO membrane.
- The carbon filter reduces chlorine, taste, and odor issues, protecting the membrane and improving the final water taste.
2.Semi-permeable membrane:
- This is the heart of the RO system. It's a thin film with microscopic pores that allows water molecules to pass through while blocking larger molecules and ions like calcium and magnesium, responsible for water hardness.
3.High-pressure pump:
- As the name suggests, this pump increases the pressure of the incoming water. This pressure is necessary to overcome the natural osmotic pressure that pushes water from the less concentrated solution (treated water) towards the more concentrated solution (untreated water) during the filtration process.
4.Restrictor valve (optional):
- This valve regulates the flow of concentrated brine solution (rejected water) that doesn't pass through the membrane. It helps maintain the optimal pressure for efficient operation.
5.Permeate tank (optional):
- This tank stores the purified water produced by the RO membrane. It provides a reserve of treated water and ensures a steady flow when needed.
6.Post-filter (optional):
- This final filter polishes the taste and odor of the RO water, further enhancing its quality.
Additional components in some systems:
- Control valve: Automates the flow of water through the system and initiates regeneration cycles (if applicable).
- Mineral remineralization cartridge (not common): Adds back minerals that may have been removed during the RO process.
By working together, these components enable reverse osmosis systems to effectively remove a wide range of contaminants, including hardness minerals, from your water supply.
- Mechanism: Uses a semi-permeable membrane to allow purified water to pass through while rejecting impurities including calcium.
- Advantages:
- Efficient and cost-effective
- Suitable for domestic and industrial applications
- Better tasting drinking water
- Reduced scale buildup
- Healthier skin and hair
- Improved appliance performance
- Disadvantages:
- Requires high water pressure
- May require pre-treatment
- Membranes need to be replaced over time
4. Emerging technology
- Electrodeionization: Uses an electric current to separate calcium and magnesium ions.
- Adsorptive nanoparticles: Uses nanoparticles to adsorb calcium and magnesium ions.
Advanced membrane processes: Such as nanofiltration reverse osmosis for high-efficiency calcium removal
5. chemical treatment for removing calcium from water:
Chemical treatment for removing calcium from water utilizes two main approaches: precipitation and chelation. Let's delve into each method:
Precipitation:
- This method aims to convert dissolved calcium ions (Ca²⁺) into an insoluble solid that can be filtered out.
- Common chemicals used include:
- Sodium carbonate (washing soda): Reacts with calcium ions to form calcium carbonate (chalk):
- Na₂CO₃ (aq) + Ca²⁺ (aq) → CaCO₃ (s) + 2Na⁺ (aq)
- Lime (calcium hydroxide): Similar reaction, but with the added benefit of increasing water pH:
- Ca(OH)₂ (aq) + Ca²⁺ (aq) → 2CaCO₃ (s) + 2OH⁻ (aq)
- The formed calcium carbonate precipitates settle out of the water or are trapped by a filter.
- Limitations:
- Primarily targets calcium carbonate, the most common form of hardness. May not be effective against other hardness minerals.
- Requires precise chemical dosing to avoid overtreatment, which can leave residual chemicals or raise water pH excessively.
Chelation:
- This method uses chelating agents like sodium citrate or EDTA (ethylenediaminetetraacetic acid) to "capture" calcium ions.
- Chelating agents have a structure that forms a ring around the calcium ion, rendering it inactive.
- Chelating agent (aq) + Ca²⁺ (aq) → Ca-Chelate (aq)
The resulting calcium chelate complex remains dissolved in water but doesn't contribute to hardness or scale formation.
Chemical treatment can be a solution for calcium removal, but its complexity, limited effectiveness, and potential downsides make it a less favorable option compared to established methods like ion exchange or reverse osmosis. Chemical treatment is generally not a recommended method for household water softening due to several drawbacks:
- Complexity: Chemical dosing requires careful measurement and monitoring to ensure effectiveness and avoid overtreating the water. Incorrect application can lead to inefficiencies or even health risks if improper chemicals are used.
- Limited Effectiveness: Chemical precipitation methods typically target calcium carbonate, the most common form of hardness. They may not be effective against other hardness minerals or contaminants.
- Secondary Issues: Chemical reactions can introduce unintended consequences. For example, some softening chemicals can increase sodium content or alter pH levels in the water.
- Disposal Concerns: Spent chemicals and precipitates from treatment may require special disposal procedures to avoid environmental contamination.
6. Comparison of methods:
Method | Advantages | Disadvantages |
Resin water softener | Efficient, cost-effective, low maintenance | Requires salt, increases sodium, not suitable for industrial applications |
Distillation | Removes almost all impurities, suitable for industrial applications | Expensive, energy-intensive, changes the taste of water |
Reverse osmosis | Efficient, cost-effective, no taste change | Requires high water pressure, pre-treatment, membrane replacement |
Emerging technologies | Under study, potential for high efficiency | High cost, early development stages |


